Call : 08045479613
GST : 27AAACN4805H1ZM
NGL Fine Chem Ltd. has started it's operation in 1981 and today, it is one of the top notch manufacturers, exporters and suppliers in the area of pharmaceutical products. At our organization, we provide a broad spectrum of products such as APIs Human Health, Finished Dosage Form, APIs Animal Health and Intermediates that are widely used in veterinary and human healthcare industries.
We have achieved certifications like ISO 9001 from SGS UK and UKAS quality management that makes us more credible in the market. Our business model is revolves around the changing demands of the clients and our capability to provided value added pharmaceutical solutions to Pharmaceutical, Chemical, Agrochemical, Electronics, Biotechnology and other industries.
Our Commitment
The commitments we have made with our clients reflected on our qualitative services and support. We have adopted four solution approaches to serve our customers in the best possible way. These approaches are:
Quality Policy
It is the policy of our company to formulate Pharmaceutical Formulations to satisfy our customers requirements, whilst complying with applicable laws and regulations. And to achieve this, NGL Fine Chem Ltd. maintains a Quality Management System, which is fully appropriate to the needs of the organization and our customers.
The quality policy is fully endorsed by our appointed quality experts, who are committed to the continuous improvement towards quality objectives and procedures. This is enabling our organization to maintain an effective and up to date Quality Management System.
Quality Assurance
The production plants at NGL Fine Chem Ltd. are designed in according to cGMP standards so to produce best quality pharmaceutical products. All the raw material are tested prior to production so to ensure that our goods meet the standards of our clients. Our quality committed behavior is reflected in all the activities from raw material procurement to the development & implementation of production methodologies to the final production examination.
Process Development
The core competence of NGL Fine Chem Ltd. is its complex multi step organic synthesis which is used for the construction of generic API's and intermediates. Our team comprises industry professionals who are consistently developing innovative technologies to serve our clients better. We have well equipped research lab, versatile pilot plant which is employed with modern analytical solutions and a kilo lab facility which enable us to provide effective pharmaceutical products to the global clients. We emphasize more on the process development and have strong connections with academia that assist us in integrating industry advances and technology.
Contract Manufacturing
NGL Fine Chem Ltd. has manufacturing plants situated in Mumbai that are designed to meet the specified requirements of Drug Regulatory Agencies.
Our current manufacturing facilities comprises-
Pilot Plant- We have a pilot plant facility for scaling up and developing new products and processes.
Our pilot plant facilities comprises:
Reaction Capabilities
We have achieved certifications like ISO 9001 from SGS UK and UKAS quality management that makes us more credible in the market. Our business model is revolves around the changing demands of the clients and our capability to provided value added pharmaceutical solutions to Pharmaceutical, Chemical, Agrochemical, Electronics, Biotechnology and other industries.
Our Commitment
The commitments we have made with our clients reflected on our qualitative services and support. We have adopted four solution approaches to serve our customers in the best possible way. These approaches are:
- Cost Effectiveness
- Product Quality
- Registration Support
- Reliability
Quality Policy
It is the policy of our company to formulate Pharmaceutical Formulations to satisfy our customers requirements, whilst complying with applicable laws and regulations. And to achieve this, NGL Fine Chem Ltd. maintains a Quality Management System, which is fully appropriate to the needs of the organization and our customers.
The quality policy is fully endorsed by our appointed quality experts, who are committed to the continuous improvement towards quality objectives and procedures. This is enabling our organization to maintain an effective and up to date Quality Management System.
Quality Assurance
The production plants at NGL Fine Chem Ltd. are designed in according to cGMP standards so to produce best quality pharmaceutical products. All the raw material are tested prior to production so to ensure that our goods meet the standards of our clients. Our quality committed behavior is reflected in all the activities from raw material procurement to the development & implementation of production methodologies to the final production examination.
Process Development
The core competence of NGL Fine Chem Ltd. is its complex multi step organic synthesis which is used for the construction of generic API's and intermediates. Our team comprises industry professionals who are consistently developing innovative technologies to serve our clients better. We have well equipped research lab, versatile pilot plant which is employed with modern analytical solutions and a kilo lab facility which enable us to provide effective pharmaceutical products to the global clients. We emphasize more on the process development and have strong connections with academia that assist us in integrating industry advances and technology.
- Alkylations
- Chlorosulfonation
- Diazotization
- Halogenation
- Hazardous / Toxic Reactions
- High temperature Reactions
- Hydrogenation
- Nitration
- Oxidations
Contract Manufacturing
NGL Fine Chem Ltd. has manufacturing plants situated in Mumbai that are designed to meet the specified requirements of Drug Regulatory Agencies.
Our current manufacturing facilities comprises-
- Plant Area: 9000 m2
- Glass Lined Reactor : 20 m3
- Stainless Steel reactors: 40 m3
- Gas Induction reactor: 3 m3
- And other equipment consisting of Lead Lined /Mild Steel Reactor:H10 m3
Pilot Plant- We have a pilot plant facility for scaling up and developing new products and processes.
Our pilot plant facilities comprises:
- Plant Area: 2000 sq.ft
- Glass Reactor: 50 liters- 5 Nos
- Stainless Steel reactors: 200 liters 400 liters
- Gas Induction reactor: 5 liters
- Other equipment comprising of Bioreactor: 2000 Liters
Reaction Capabilities
- Alkylations
- Chlorosulfonation
- Diazotization
- Halogenation
- Hazardous / Toxic Reactions
- High temperature Reactions
- Hydrogenation
- Nitration
- Oxidations

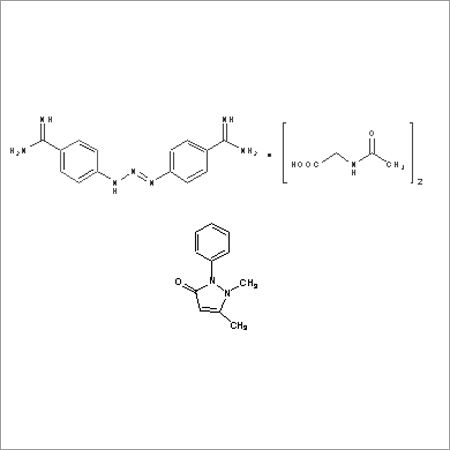
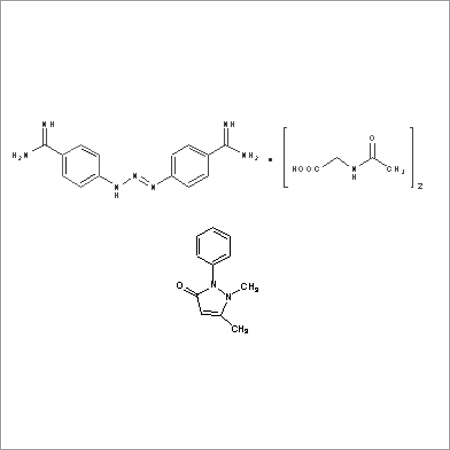
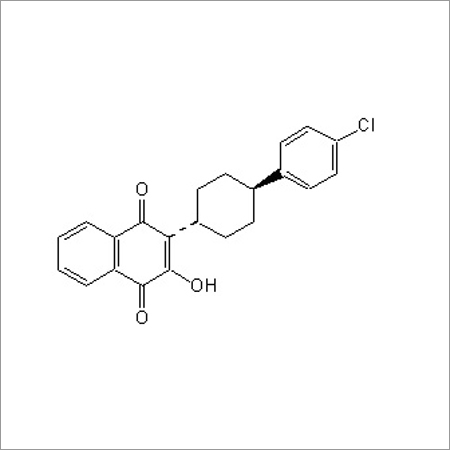
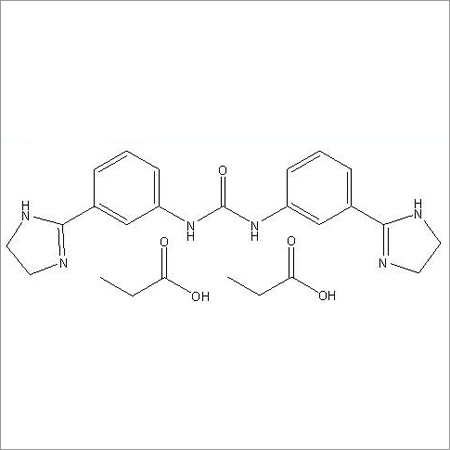
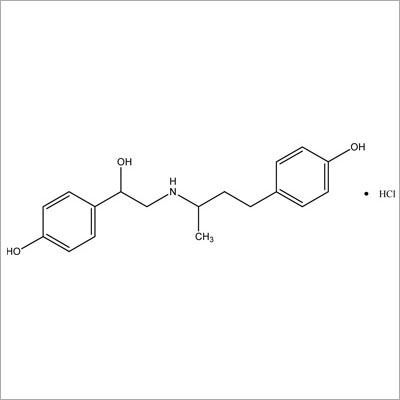
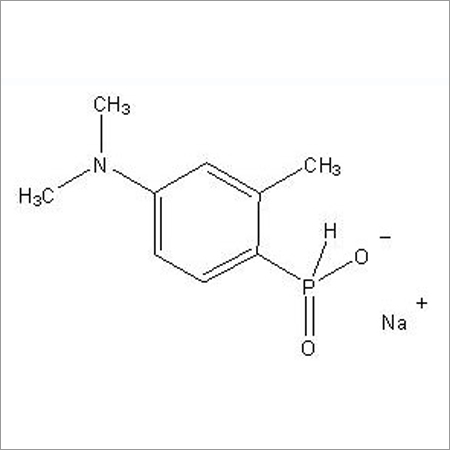
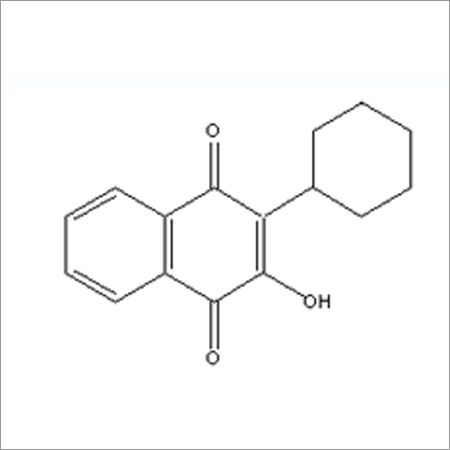
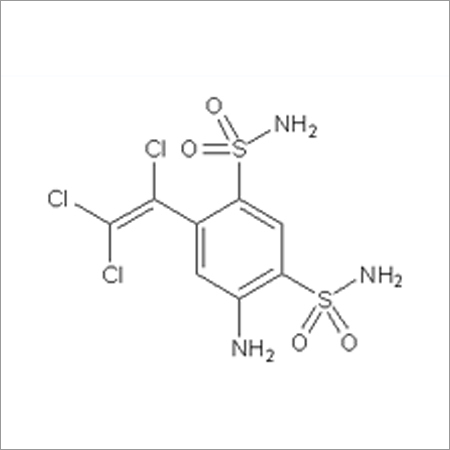
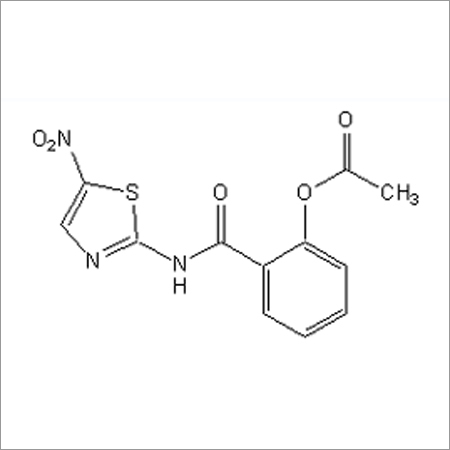
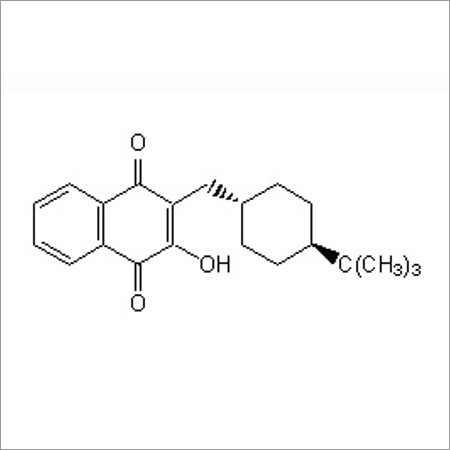
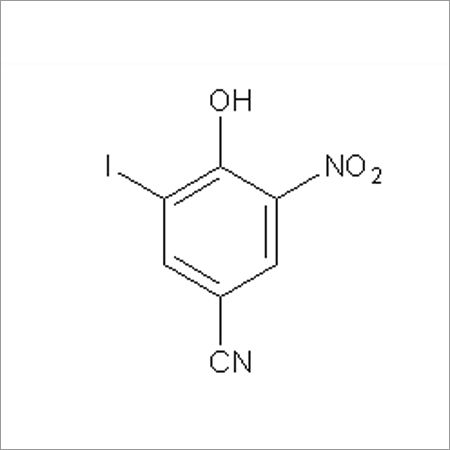
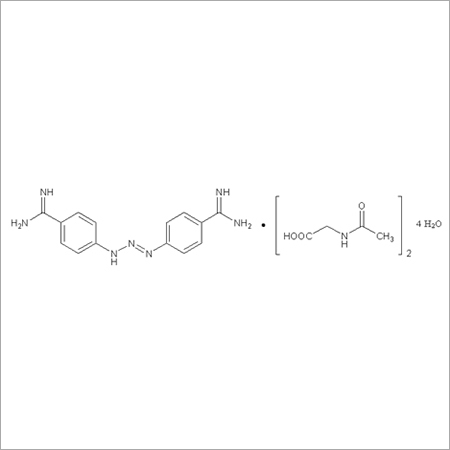
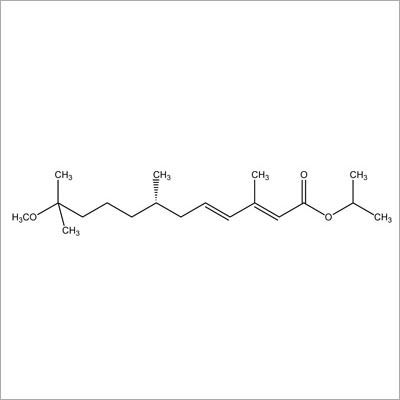
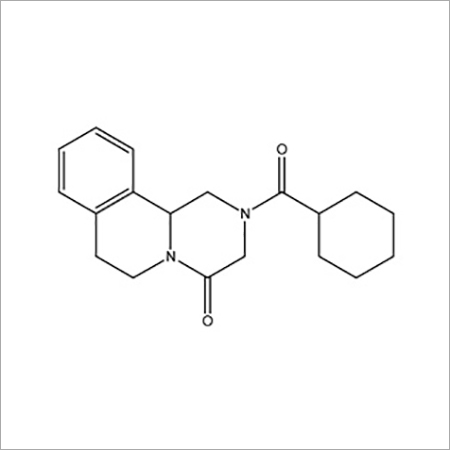
Most Popular Products
 |
NGL FINE CHEM LTD.
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |

 Send Inquiry
Send Inquiry